Sources Of Aldehydes And Ketones
Sources Of Aldehydes And Ketones. The difference between carboxylic acid derivatives and aldehydes and ketones is that there is a group containing a negatively charged heteroatom (usually oxygen, nitrogen or sulfur), which is directly connected to the carbonyl carbon atom. The compounds in the top row are found chiefly in plants or microorganisms;
![Reduction of Aldehydes and Ketones [PPT Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20180902/56814944550346895db68e48/document-13.png?t=1627928451)
![Reduction of Aldehydes and Ketones [PPT Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20180902/56814944550346895db68e48/document-13.png?t=1627928451) Reduction of Aldehydes and Ketones [PPT Powerpoint] from vdocuments.mx
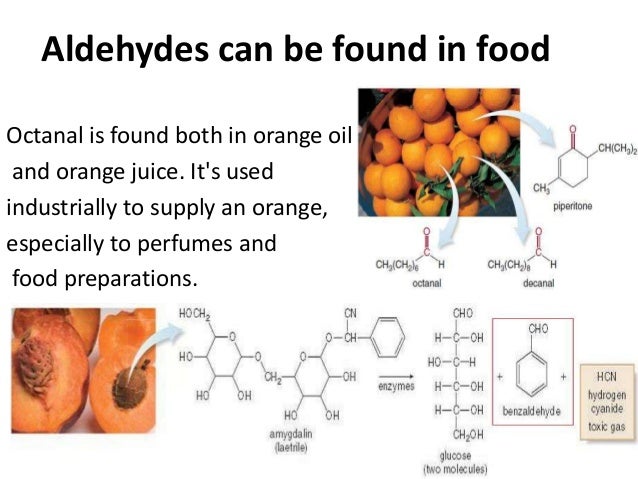
Reduction of Aldehydes and Ketones [PPT Powerpoint] from vdocuments.mxComplicated ketones can be made by the oxidation of alcohols, which in turn can be made from reaction of a grignard and an aldehyde. Aldehydes and ketones are present in nature in several forms, such as cinnamaldehyde lending its unique fragrance to cinnamon bark, and the presence of vanillin provides a unique fragrance to vanilla, which is the basis of vanilla essence. 2 aldehyde and ketone containing molecules isolated from animal sources.
![Chapter 9 Aldehydes and Ketones [PPTX Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20171215/5681671f550346895ddba020/document-12.png?t=1621853755)
Aldehydes and ketones are the two functional groups that share a lot of similarities. Example are shown in the following diagram.
![Reduction of Aldehydes and Ketones [PPT Powerpoint]](https://static.documents.pub/img/1200x630/reader012/image/20180902/56814944550346895db68e48.png?t=1606757912)
They both have a carbonyl functional group, but the main difference is in the atoms bonded to the central carbon. The application of this method to determine the concentration of these compounds in the atmospheres of buildings is described and the results compared with those obtained using chromotropic acid or mbth.
Tollens' reagent, which is a mixture of silver nitrate and ammonia, oxidizes the aldehyde to a carboxylic acid. Aldehydes aldehydes are organic compounds that are widespread in nature.

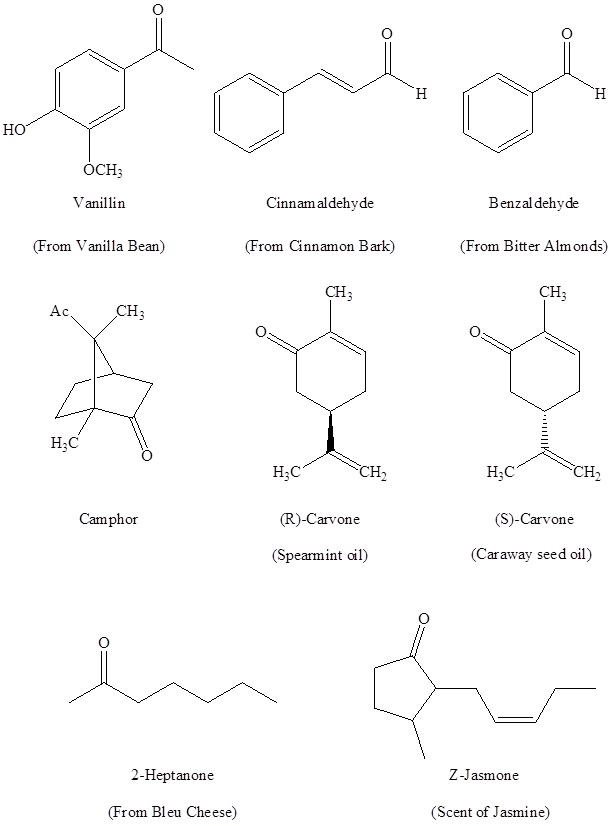
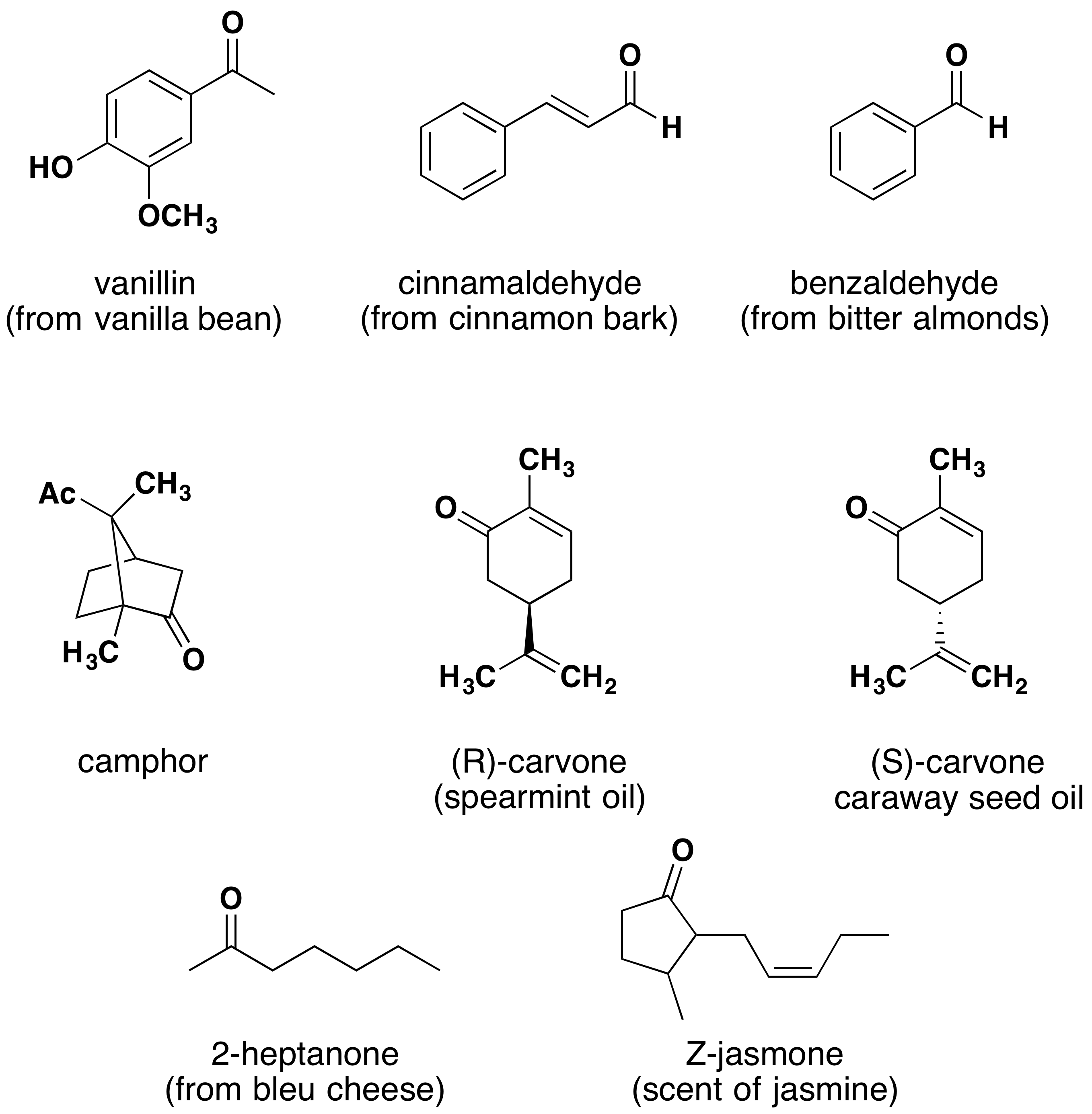
The compounds in the figure 1 are found chiefly in plants or microorganisms and those in the figure 2 have. Aldehydes & ketones concepts are essential to learning for every level in chemistry courses.
![Reduction of Aldehydes and Ketones [PPT Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20180902/56814944550346895db68e48/document-13.png?t=1627928451)
Aldehydes and ketones are widespread in nature and are often combined with other functional groups. The silver ion is, concomitantly, reduced to metallic silver.
Due to high demand and limited spots there is a waiting list. In aldehydes, the carbon atom in the carbonyl group is bounded to a hydrogen and one carbon atom while in ketones it is bound to two other carbon atoms.

Compounds such as cinnamaldehyde (cinnamon bark), vanillin (vanilla bean), citra (lemongrass), helminthosporal (a fungal toxin), carvone (spearmint and caraway), camphor (camphor trees) are found chiefly in microorganisms or plants. Aldehydes and ketones are present in nature in several forms, such as cinnamaldehyde lending its unique fragrance to cinnamon bark, and the presence of vanillin provides a unique fragrance to vanilla, which is the basis of vanilla essence.
![Chapter 9 Aldehydes and Ketones [PPTX Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20171215/5681671f550346895ddba020/document-6.png?t=1621853755)
Aldehydes and ketones are the compounds containing the carbonyl group in their molecules. Aldehydes and ketones are present in nature in several forms, such as cinnamaldehyde lending its unique fragrance to cinnamon bark, and the presence of vanillin provides a unique fragrance to vanilla, which is the basis of vanilla essence.

Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. The application of this method to determine the concentration of these compounds in the atmospheres of buildings is described and the results compared with those obtained using chromotropic acid or mbth.

Aldehydes are organic compounds that are widespread in nature. Sources and concentrations of aldehydes and ketones in indoor environments in the uk
Ketones are more polar than aldehydes. Silver ion is a weak oxidizing agent;

Aldehydes and ketones in combination with other functional groups are widely available in nature. Which is more polar aldehyde or ketone?
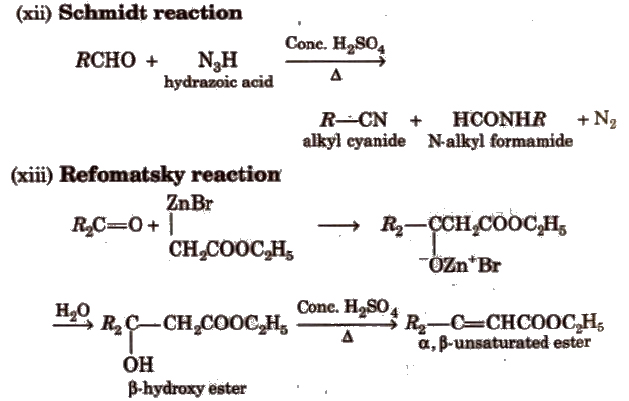
Ch18 ketones and aldehydes (landscape) page 6 syntheses of the aldehydes and ketones (recap?) from alcohols (ch 11) secondary alcohols are readily oxidized to ketones by chromic acid (or kmno 4). Tollens' reagent, which is a mixture of silver nitrate and ammonia, oxidizes the aldehyde to a carboxylic acid.
![Chapter 9 Aldehydes and Ketones [PPTX Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20171215/5681671f550346895ddba020/document-7.png?t=1621853755)
Which is more polar aldehyde or ketone? Silver ion is a weak oxidizing agent;

The compounds in the figure 1 are found chiefly in plants or microorganisms and those in the figure 2 have. Aldehydes and ketones are widespread in nature including plants, microorganisms, animals, and humans.
![Chapter 9 Aldehydes and Ketones [PPTX Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20171215/5681671f550346895ddba020/document-2.png?t=1621853755)
Winner of the royal society of chemistry schools education award in 2016, i produce teaching & assessment resources for chemistry & much more. Making use of the latest software i produce all kinds of interactions, iquizzes and simple animations.
![Reduction of Aldehydes and Ketones [PPT Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20180902/56814944550346895db68e48/document-23.png?t=1627928451)
Example are shown in the following diagram. We hope you will find this very useful for your regular exam preparations and entry tests at undergraduate levels, particularly for medical and engineering students (mdcat, ecat, nust,.
![Chapter 9 Aldehydes and Ketones [PPTX Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20171215/5681671f550346895ddba020/document-5.png?t=1621853755)
Aldehydes and ketones are widespread in nature, often combined with other functional groups. Aldehydes and ketones are widespread in nature and are often combined with other functional groups.

There are many essential uses of aldehydes and ketones and they form an inevitable part of many industrial processes. Compounds such as cinnamaldehyde (cinnamon bark), vanillin (vanilla bean), citra (lemongrass), helminthosporal (a fungal toxin), carvone (spearmint and caraway), camphor (camphor trees) are found chiefly in microorganisms or plants.
![Reduction of Aldehydes and Ketones [PPT Powerpoint]](https://reader012.vdocuments.mx/reader012/slide/20180902/56814944550346895db68e48/document-15.png?t=1627928451)
Example are shown in the following diagram. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation.
Aldehydes And Ketones Are Present In Nature In Several Forms, Such As Cinnamaldehyde Lending Its Unique Fragrance To Cinnamon Bark, And The Presence Of Vanillin Provides A Unique Fragrance To Vanilla, Which Is The Basis Of Vanilla Essence.In aldehydes, the carbon atom in the carbonyl group is bounded to a hydrogen and one carbon atom while in ketones it is bound to two other carbon atoms. Silver ion is a weak oxidizing agent; Aldehydes and ketones in combination with other functional groups are widely available in nature.
Ketone Is A Class Of Organic Compounds That Are Characterized By The Presence Of A Carbonyl Group (Co) As A Functional Group.You will be notified when your spot in the trial session is available. An aldehyde is an organic compound. Aldehydes and ketones are widespread in nature and are often combined with other functional groups.
Aldehydes Are Very Easily Oxidized And Are Essentially Unique In Being Able To Reduce Silver Ion To Silver Metal.They both have a carbonyl functional group, but the main difference is in the atoms bonded to the central carbon. When these compounds are brought to their. The tollens' test is a reaction that is used to distinguish aldehydes from ketones, as aldehydes are able to be oxidized into a carboxylic acid while ketones cannot.
Aldehydes Are Also Oxidized By Tollens' Reagent, A Substance That Contains Ag+1.Complicated ketones can be made by the oxidation of alcohols, which in turn can be made from reaction of a grignard and an aldehyde. Those in the bottom row have animal origins. While aldehydes have an r group chain of hydrocarbon substituents and a hydrogen attached to the central carbon, ketones have r and r' substituents attached to.
Making Use Of The Latest Software I Produce All Kinds Of Interactions, Iquizzes And Simple Animations.Thus, due to the structural similarity, aldehydes and ketones have many reactions that are the same for the both functional groups. There are many essential uses of aldehydes and ketones and they form an inevitable part of many industrial processes. An aldehyde has at least one hydrogen connected to the carbonyl carbon.
Komentar
Posting Komentar